Nowadays we are all so aware and well informed about skincare. We know what products suit our skin type, which specific ingredients our skin needs, which ingredients we rather avoid. We are more informed than ever. But one thing that still tends to get overlooked and doesn’t get the attention it deserves is the skin’s first line defense barrier; the acid mantle. Today’s post is all about this protective layer. I will try to explain the importance of this barrier, how the skin's pH is involved, and how to keep it all balanced.
The Acid Mantle is a Protective Barrier
The acid mantle is a very thin, slightly acidic film that covers the surface of the skin. It is composed of secretions from sweat and sebaceous glands and fatty acids provided by beneficial microflora residing on the skin. The acid mantle acts as a barrier to harmful bacteria, viruses, and other contaminants that might penetrate the skin. When the acid mantle is disrupted, the skin loses it’s natural balance. Essential epidermal lipids can no longer be synthesized, which results in the skin losing water and eventually drying out. The skin basically loses it’s protective barrier. When the barrier is lost, the skin is less resilient and more sensitive to environmental aggressors. It becomes dry, sensitive, and susceptible to infections and diseases like Atopic Dermatitis and Rosacea.
The Acid Mantle and the Skin's pH
The key element to the acid mantle is acidity. This is where pH comes into play. The pH level of the skin describes how acidic or alkaline it is. A pH of 7 is considered ‘neutral’. A pH below 7 is considered ‘acidic’ and everything above 7 is considered alkaline. Healthy skin has a pH of ~4.5-5.5, so slightly acidic. An acidic pH is vital to keep the acid mantle intact. When the skin’s pH starts to elevate, the acid mantle slowly degrades and the protective barrier gets lost, giving a free pass to contaminants to penetrate the skin. 
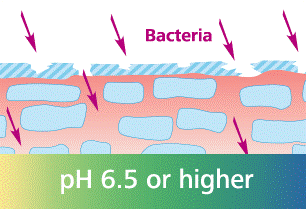
What is disrupting the skin’s pH?
So what can cause the skin’s pH to rise and disrupt it’s protective layer?
The main environmental triggers that can strip the skin from it’s protective barrier include:
-Changes in temperature and humidity
-Dirt and pollution
-Washing too frequently
-Alkaline cosmetics (especially cleansers)
Alkaline cosmetics typically refer to cleansers and detergents used in cleansers (pH 9-10). One such detergents used in cleansers is sodium lauryl sulfate (SLS). SLS gets rid of oil and dirt, but it’s so harsh that it also strips away the skin’s acid mantle. Most Korean skincare products (mostly cleansers) avoid this ingredient for that reason.
The best advice I want to give you is from my own experience: don't wash your face more often than necessary! Even the water you use to wash your face can dry out the skin a bit. Also, don't use products that are too acidic for your face. They are also bad for your skin's protective acid mantle. Do not spray lemon juice (pH 2) on your face. Your skin will not be happy with this. Choose products with a pH between 3 and 6.5.
References
International Journal of Cosmetic Science, September 2006, pages 359-370.
Dermatology Clinics, January 1996, pages 23-27.







 Trending
Trending
 LW Blog
LW Blog










